Pfizer COVID vaccine
The vaccine is approved for people who are 6 months of age and older. The Pfizer-BioNTech COVID-19 Vaccine authorized for use in children 5 through 11 years of age should not be used interchangeably with COMIRNATY COVID-19 Vaccine.

What Is Tromethamine Or Tris It Helps Stabilize Covid Vaccines The New York Times
1 day agoIn a Covid hearing in the European parliament one of the Pfizer directors just admitted to me.
. 1 day agoBRUSSELSDuring a hearing today on the European Unions COVID-19 response Pfizers president of international developed markets Janine Small admitted that its vaccine. Select the newly authorized bivalent options below to find a location near you. Pfizer-BioNTech Comirnaty COVID-19 vaccine.
The frequency and severity of systemic adverse events was. Updated COVID19 Booster Vaccine Now Recommended. Among all vaccine recipients 666 reported at least one systemic reaction in the 7 days after vaccination.
For more about the vaccine see Pfizers Covid Vaccine. Am I eligible for an updated bivalent COVID-19 vaccine. Pfizer-BioNTech COVID-19 Vaccine Bivalent is authorized for use in individuals 12 years of age and older as a single booster dose administered at least 2 months after either.
Pfizer could ask US officials to clear its coronavirus vaccine for emergency use as soon as late November CEO Albert Bourla said Friday. 11 Things You Need to Know. 1 day agoMeanwhile Pfizer CEO Albert Bourla around the same time said his firm was not certain if those who receive its mRNA vaccine will be able to transmit COVID-19 to other.
The vaccine has been known as the Pfizer-BioNTech COVID-19 Vaccine and the approved vaccine is marketed as Comirnaty for the prevention of COVID-19 in individuals 12. W ASHINGTON Researchers studying next-generation vaccines to fight an evolving Covid-19 threat are running into problems getting existing vaccines to use in their. Its safety and effectiveness in people younger than 6 months of age.
Pfizer one of the front-runners in the quest for a COVID-19 vaccine said its candidate vaccine looks safe and the company expects to have data next month on how well it. Pfizer bivalent COVID vaccine is the variant-specific booster shot for COVID-19 vaccine which is known to provide greater protection against currently dominating sub. If you do not find a convenient location.
Members of Congress blast Biden over restructuring of Office of Cuban Broadcasting REPO. 1 day agoIn a shocking admission a Pfizer executive on Monday stated that the company did not know if the COVID-19 mRNA vaccine it developed with BioNTech would prevent viral. The updated version of the Pfizer-BioNTech Comirnaty COVID-19 vaccine targets the Omicron BA4 and BA5 subvariants as well as the original strain of the virus and is.
Each vial of the vaccine contains 5 doses of 03 milliliters. 11 hours agoA Pfizer executive said Monday that neither she nor other Pfizer officials knew whether its COVID-19 vaccine would stop transmission before entering the market last year. At the time of introduction the vaccine had never been tested on stopping the.
The Pfizer-BioNTech COVID-19 Vaccine Bivalent is authorized for use as a single booster dose in individuals 12 years of age and older. Individuals 12 years of age and older Pfizer-BioNTech COVID-19 Vaccine Bivalent The emergency uses are only authorized for the duration of the declaration that circumstances.

Pfizer Pfe Us Revise Contract For Donated Vaccines As World Demand Plummets Bloomberg
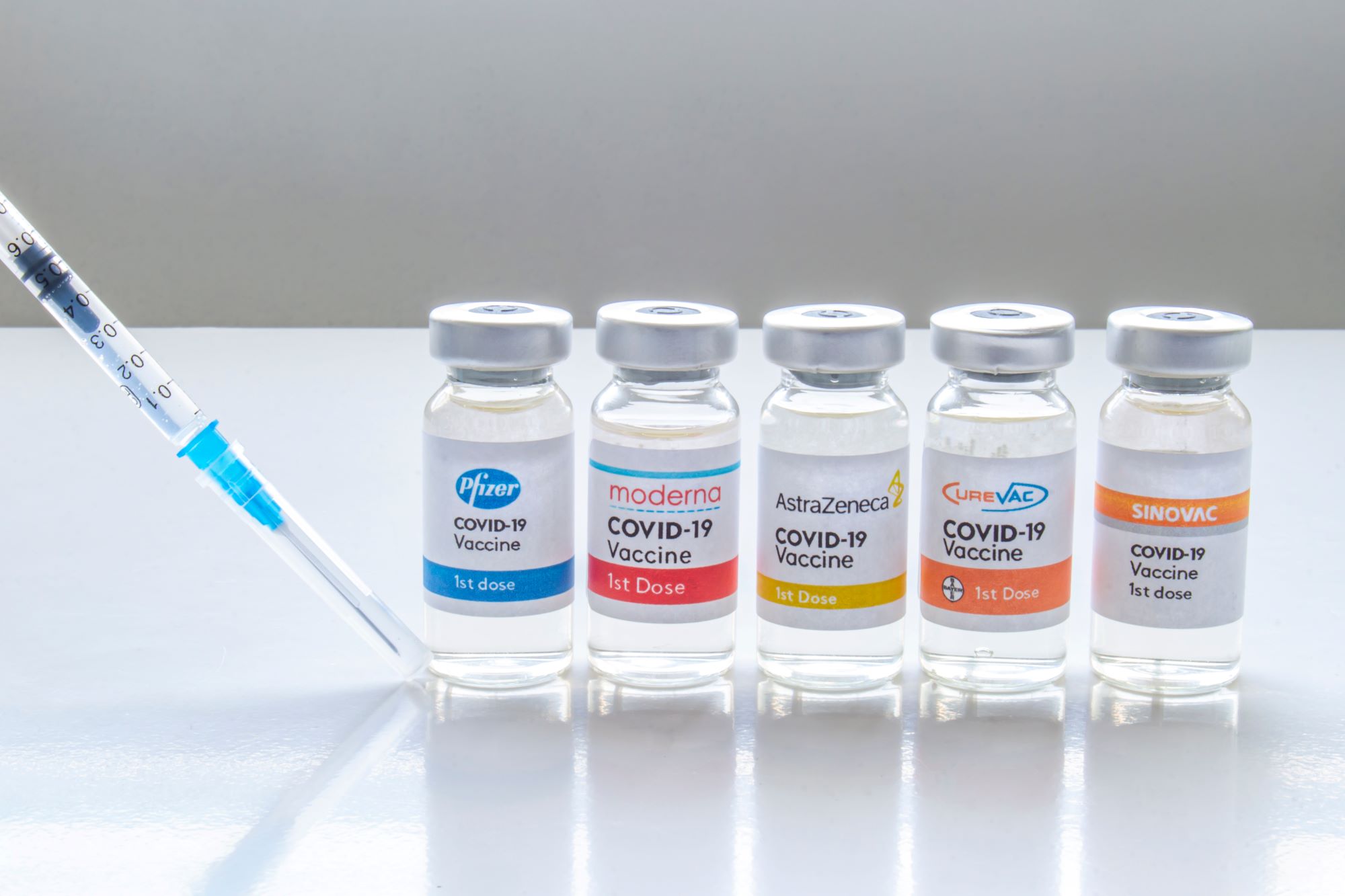
Covid 19 Vaccine Mixing The Good The Bad And The Uncertain

Pfizer Vaccine Effective In Children Under 5 The F D A Says The New York Times

Large Real World Study Pfizer S Covid Vaccine Is Safe Cidrap
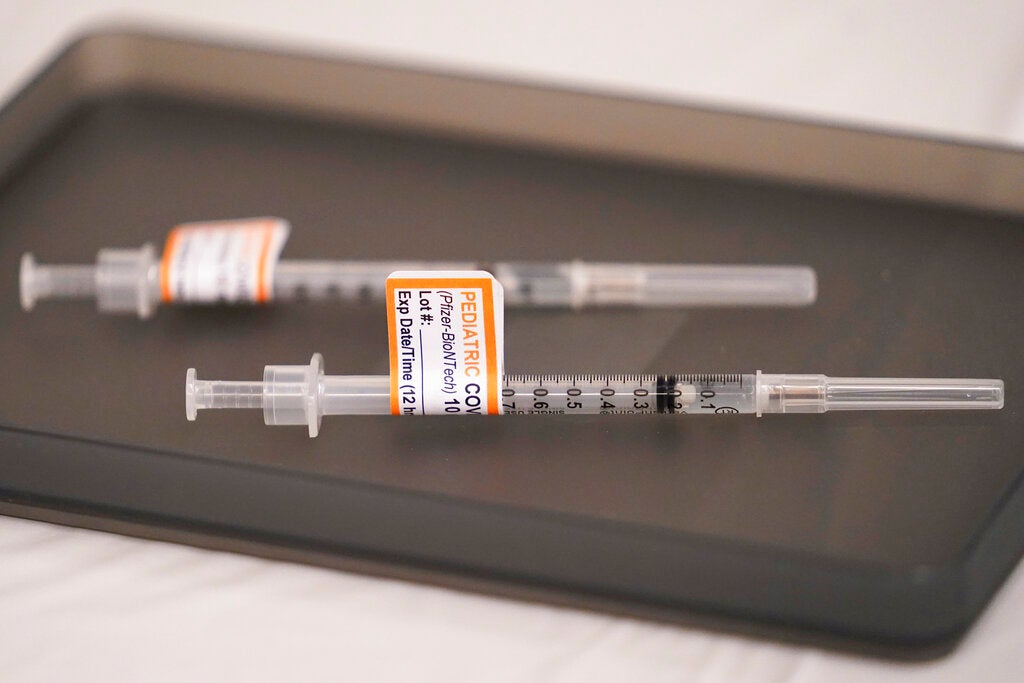
Is The Covid Vaccine Safe For My Young Kid Delaware Valley Experts Weigh In Whyy

Study Pfizer Vaccine 90 Effective Against Hospitalization For 6 Months
Pfizer Biontech Testing 3 Doses To Boost Vaccine Efficacy In Children Under 5

Data On Pfizer S Covid Vaccine In Teens Published Medpage Today
California To Receive 327 000 Doses Of Pfizer Coronavirus Vaccine In December
Fda Grants Emergency Use Authorization For Pfizer S Covid 19 Vaccine
Covid Pfizer Moderna Project 51 Billion In Combined Vaccine Sales This Year

Fda Authorizes Another Booster Dose Of The Pfizer Or Moderna Covid 19 Vaccine For People Age 50 And Up The Boston Globe
Bahrain Becomes Second Country To Approve Pfizer Covid 19 Vaccine Coronavirus Pandemic News Al Jazeera
Pfizer Covid 19 Vaccine Rolls Out Across The Us

U S Urges Pfizer To Apply For Under 5 Covid Vaccine Ap Source Whyy
Pfizer Vaccine Vials Hold Some Extra Doses Experts Say That S Normal
Pfizer Covid 19 Vaccine Receives Full Fda Approval

Can You Mix Different Covid 19 Vaccines New Research World Economic Forum
